Several Cancer Center at Illinois (CCIL) and IGB members are joining forces with scientists from the Mayo Clinic and Georgetown University on an expansive project targeting improved treatment for glioblastoma (GBM), the most aggressive form of brain cancer. The team, led by Brendan Harley (RBTE leader/EIRH), professor of chemical and biomolecular engineering, recently received a $3M grant from the National Cancer Institute (NCI) for their research which will unite the cell biology, bioengineering, and chemistry behind cancer drug development.
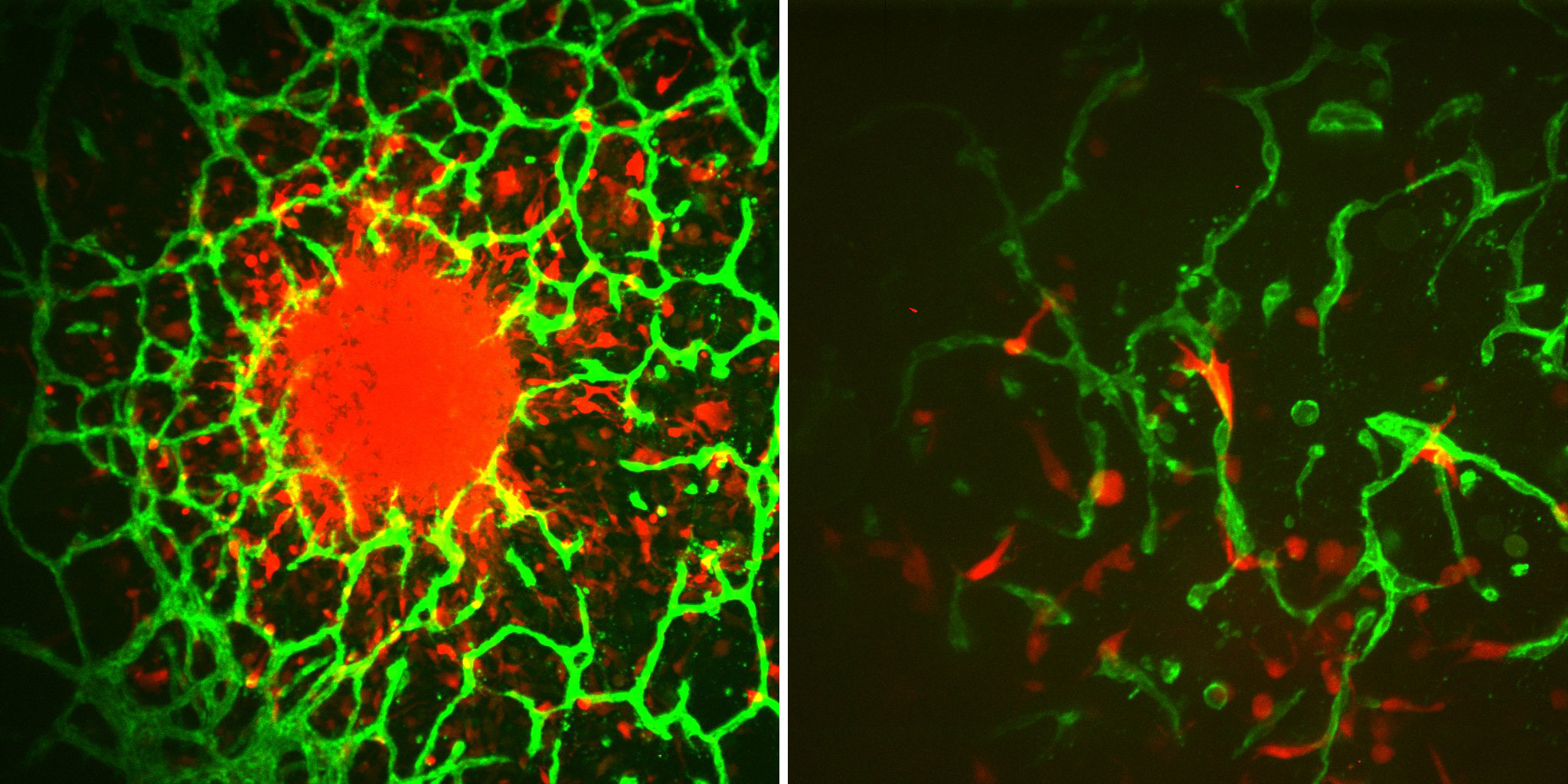
Glioblastoma is historically difficult to treat, with only a 25% survival rate over five years. When a cancer patient is diagnosed with GBM, they will most likely be undergoing surgery to remove the tumor – made significantly more difficult due to its sensitive location.
“Glioblastoma patients tend to have tumor re-occurrences within six to seven months of the surgery, and it’s within centimeters of the tumor margin,” Harley said. “It’s caused by the cancerous cells that could not be removed. This issue is what got our team really interested in how we can develop better therapeutic treatments.”
Harley, also a CCIL Research Program Theme leader, is an expert in building tissue microenvironments, using bioengineering techniques to re-create the brain tissue. His lab is partnering with the Mayo Clinic and Rebecca Riggins, associate professor of oncology at Georgetown, to provide cell lines engineered to be resistant to temozolomide (TMZ), the primary chemotherapy used to treat GBM today.
Patient-derived cell lines, provided by Dr. Jann Sarkaria of Mayo Clinic who is another investigator on this project, provide another key element of the work. They are developing a biomaterial that simulates the vascular environment in the brain in order to understand how the tumor microenvironment can promote GBM cell resistance to drugs.
“When you treat a patient with TMZ, it causes damage to the DNA in the cancerous cells, causing them to die. However, these cells are able to avoid this damage in a number of ways, but a primary way is through MGMT [a DNA repair enzyme],” Harley said. “TMZ induces a mutation to the cancerous cell’s DNA to destroy it, but MGMT can reverse that.”
“So if a patient is MGMT-positive, they are more likely to be resistant to TMZ. Unfortunately, more than 60% of GBM patients are MGMT-positive, so the current frontline drug is given to GBM patients who have the primary resistance to the treatment living in their bodies,” Harley said.
Harley and his team have shown that the brain vasculature mimics they have developed provide new insight into MGMT resistance. If you are MGMT-negative, you have a two-year survival rate of 50%, but if you are MGM- positive, you only have a two-year survival rate of 15%.
During a separate project, Harley and Paul Hergenrother (ACPP leader/MMG), professor of chemistry and the CCIL’s Deputy Director, realized that Hergenrother’s expertise in cancer therapeutics could aid the team in addressing MGMT.
“Since we knew the high level of resistance because of MGMT, my lab thought that we could design a compound that applies another mutation to the DNA that would not get removed, as well as something that gets into the brain more efficiently,” Hergenrother said. “The Harley lab will develop more predictive models, which will help us to evaluate the success of the cancer agents we create at a more rapid pace.”
The research team will explore how they can create a mutated version of TMZ that MGMT cannot remove, allowing for this new version of TMZ to successfully destroy cancerous cells. This grant is a part of the NCI’s Cancer Tissue Engineering Collaborative which will support the team’s efforts to better understand the physiological processes driving cancer progression and drug resistance.
“This program is committed to supporting research groups that are pushing the boundaries of tissue engineering technologies for cancer,” Harley said. “It connects us to both collaboration opportunities as well as what challenges our colleagues are facing so that we can work together to address them.”
Harley is the Robert W. Schaefer Professor of Chemical and Biomolecular Engineering. Hergenrother is the Kenneth L. Rinehart Jr. Endowed Chair in Natural Products Chemistry and the Director of the NIH Chemistry-Biology Interface Training Program.

