New microscope discovery combines AI to detect disease, improve treatment
A newly developed laser source and microscope are helping researchers better understand and search for biomarkers indicative of cancer and other diseases, offering new promise for early detection and treatment plans.
The study, co-authored by Carle Illinois College of Medicine professors Stephen Boppart and Saurabh Sinha (IGB Director of Computational Genomics, BSD/CABBI/GNDP/GSP), including researchers in Boppart’s Biophotonics Imaging Laboratory at the University of Illinois Urbana-Champaign, was recently published in Cancer Research and featured on the cover.
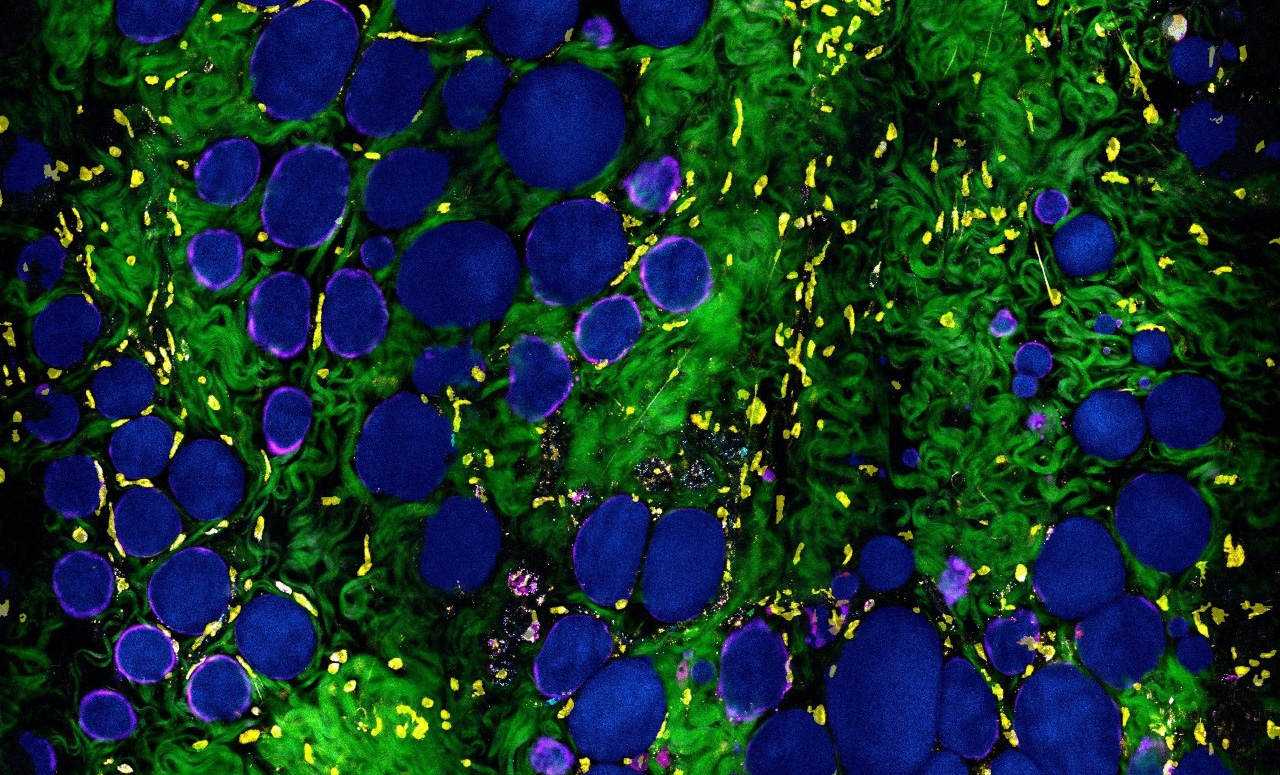
The innovative laser source and microscope collects multiple optical signals/channels from molecular, cells, and tissues without having to add any stains or dyes. “The multimodal imaging approach allows us to visualize the dynamic cells and tissues without disturbing their functions, and not only distinguish and classify different cell types, but also characterize their dynamics and metabolic profiles,” said Stephen Boppart, Carle Illinois’ Executive Associate Dean and Chief Diversity Officer.
In the study, researchers use the new microscope, combined with AI/deep-learning (deep-profiling) algorithms to investigate the tumor microenvironment and search for clues and biomarkers indicative of cancer. “This work not only automates the examination of tissue images, which is something that deep learning algorithms are being increasingly used for, it also shows how information gleaned automatically can reveal important biological features of the tumor microenvironment,” said Saurabh Sinha, Founder Professor and Willett Faculty Scholar in The Grainger College of Engineering’s Department of Computer Science.
The computational framework can characterize the complexity and heterogeneity of the tumor microenvironment and differentiate it from the normal state. The result opens possibilities for better representation and understanding of the evolving cancer landscape. “This is not only important for finding diagnostic biomarkers of cancer, but also for improving our understanding of the complex and dynamic changes that take place during carcinogenesis,” explained Boppart.
The increasing use of AI methods to identify patterns in data and images is a unique component in the advancement of this technology and research. “AI methods will continue to aid in diagnosing disease and evaluating outcomes for patients, as well as interpret and predict healthcare analytics for improving processes and efficiencies,” said Boppart.
The AI methods used in this study identify the patterns that emerge through the exploration of the relationships between cells and extracellular vesicles (EVs), improving the ability to detect, image, and characterize EVs, which have shown previously to be biomarkers of cancer.
“By exploring laser-tissue interactions and AI algorithms, we showed a way to capture the intrinsic cellular contrast and to digitally stain and phenotype different cell types in living tissues without physically staining it. This brings great value for potentially advancing living tissue diagnosis and assessment in clinical settings,” said Sixian You (Bioengineering Ph.D. ’19), lead author and assistant professor at Massachusetts Institute of Technology.
The journal Cancer Research is the most frequently cited cancer journal in the world and is the journal for the American Association for Cancer Research (AACR).
This research was supported in part by grants from the National Institutes of Health. Lead author, Sixian You, was supported by McGinnis Medical Innovation Graduate Fellowship through the bioengineering department at the University of Illinois Urbana-Champaign.
The paper “Label-Free Deep Profiling of the Tumor Microenvironment” is available online.
Stephen Boppart is also affiliated with The Grainger College of Engineering’s Department of Electrical and Computer Engineering and Department of Bioengineering, the Cancer Center at Illinois, and the Beckman Institute for Advanced Science and Technology.
Saurabh Sinha is also affiliated with The Grainger College of Engineering’s Department of Computer Science and the Cancer Center at Illinois.
