Mitochondrial protein plays key role in glioblastoma and therapeutic resistance

From left, Brendan Harley, Rex Gaskins, Andrew Steelman, and Payton Haak studied the role of the mitochondrial protein CHCHD2 in glioblastoma. / Julia Pollack
Glioblastoma is the most common type of brain tumor that affects adults and, unfortunately, still remains incurable. In a new study, researchers have demonstrated that a specific mitochondrial protein plays an important role in glioblastoma, and can therefore be used as a potential target to reduce tumors.
“Glioblastoma is notorious for its lethality. One of the major challenges is that it spreads invasively throughout the brain. We’re interested in understanding what drives this process in order to identify new therapeutic strategies,” said Brendan Harley (RBTE leader/EIRH), the Robert W. Schaefer Professor of Chemical and Biomolecular Engineering.
In the current study, the researchers focused on the mitochondrial coiled-coil-helix-coiled-coil-helix domain containing protein 2—also known as CHCHD2. The complicated name refers to the structure of the protein, whose subunits are coiled together like rope strands.
The researchers first looked at The Cancer Genome Atlas glioblastoma database to see whether they could spot any patterns that related CHCHD2 levels to cancer. Out of 577 samples, they found that the CHCHD2 genes had higher expression in tumor cells, compared to non-tumor tissue, and was higher in advanced cases of glioblastoma.
“We also learned that in humans the gene encoding CHCHD2 was closely linked to the gene encoding the epidermal growth factor receptor, or EGFR, on chromosome 7. A mutated version of this protein is found in over 50% of glioblastoma patients,” said Rex Gaskins (RBTE), the Keith W. and Sara M. Kelley Endowed Professor of Immunophysiology in Animal Sciences and corresponding author of the study.
When the results from the database showed that CHCHD2 expression was highest in the patients who harbored the mutation known as EGFRvIII, the researchers realized that understanding the interaction between these two proteins can be crucial to understanding glioblastoma progression.
To confirm their hypothesis, the researchers looked at the effects of CHCHD2 on tumor growth in mice. They compared mice that had CHCHD2 and the mutated version of EGFR to mice that did not contain CHCHD2, but still had mutated EGFR. The first group of mice survived for an average of 17 days, whereas the second group survived for 25 days. The researchers found that the mice that died earlier had more tumor growth infiltrating the surrounding brain tissue.
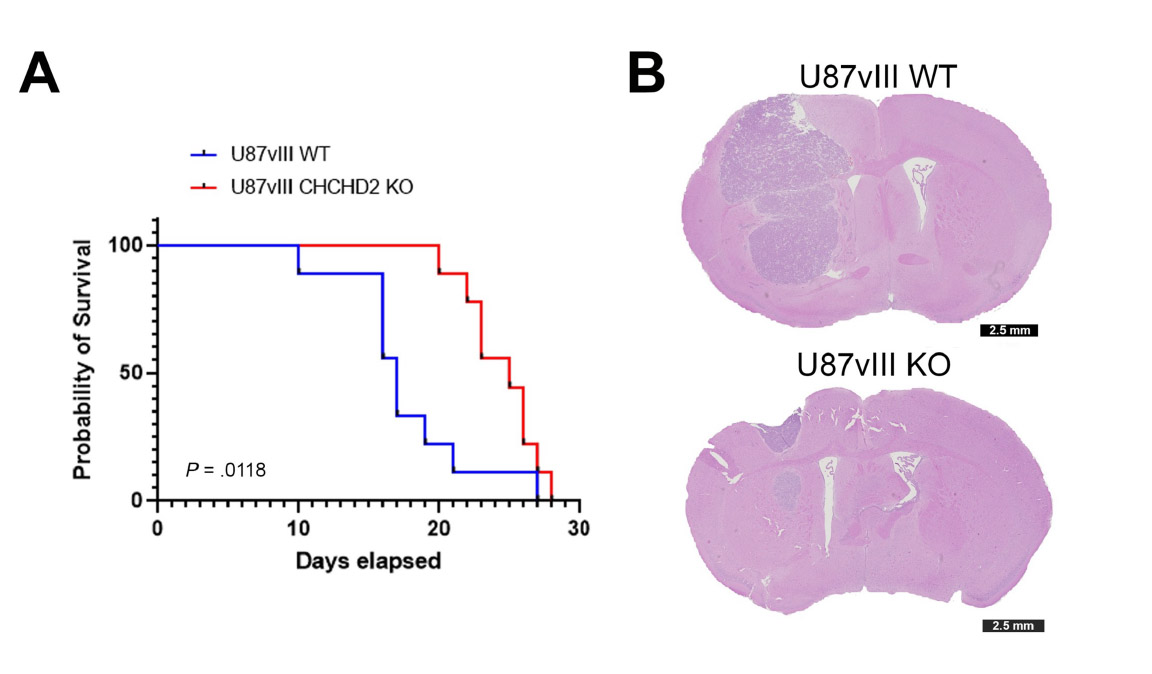
Although it is evident that these proteins are interacting with each other, it is unclear how they affect glioblastoma progression. The authors have proposed several mechanisms, based on their experiments.
The first possibility is that CHCHD2 decreases the sensitivity of mutated EGFR to cytotoxic drugs. Patients with glioblastoma only survive for 15-20 months, despite being subjected to an arsenal of treatment procedures, including chemotherapy with the drug temozolomide. The researchers used this drug and tested cells that had the mutated EGFR protein and CHCHD2 and cells that only had EGFR. They saw that when cells did not contain CHCHD2, they were more sensitive to temozolomide. The researchers believe that this result highlights CHCHD2 as a potential therapeutic target.
Another possibility is that CHCHD2 affects how EGFR stimulates glioblastoma invasion. Using a hydrogel to replicate the tumor microenvironment in the brain, the researchers showed that cells with both CHCHD2 and mutated EGFR were able to grow and invade the surrounding area. This invasion was especially pronounced when the level of oxygen was lower, a condition commonly experienced by glioblastoma cells as they invade from the tumor into the brain.
The data also demonstrate that CHCHD2 profoundly affects cellular metabolism, which the researchers showed using different biochemical tests. “It’s a multifaceted paper that used patient data, human cell lines, and a mouse model of glioblastoma,” Gaskins said. “All of our data are aligned and we can pursue any one of these mechanisms to see if it explains what is happening in the tumors.”
The researchers are interested in figuring out why the invasive capacity of these cells change, especially under low-oxygen conditions. They also want to test whether these results hold true in other types of glioblastomas, which may not have a mutated EGFR.
“This study suggests that the role of CHCHD2 in glioblastoma progression was underappreciated, but it might be a significant new target as we try to improve clinical outcomes,” Harley said. “The idea that this protein can change behavior based on its environment is valuable. We hope that researchers can start identifying new therapeutic strategies based on this key protein.”
The paper “CHCHD2 mediates glioblastoma cell proliferation, mitochondrial metabolism, hypoxia induced invasion and therapeutic resistance” was published in the International Journal of Oncology and can be found at https://pubmed.ncbi.nlm.nih.gov/37654190/. The work was funded by the NIH, and the Department of Chemical and Biomolecular Engineering and the Cancer Center at Illinois.
The work was led by Jan Lumibao and Jee-Wei Emily Chen, former trainees in the NIH-funded Tissue Microenvironment Training Program at Illinois, as well as current animal sciences graduate student Payton Haak.
