Stop Making Cells: Study Advances Knowledge about Cell Regeneration
Dr. Wenyan Mei (MME) in the Department of Comparative Biosciences recently published a paper in the journal Nucleic Acids Research called “PTBP1 controls intestinal epithelial regeneration through post-transcriptional regulation of gene expression.” The work was done in collaboration with other researchers across the University of Illinois campus.
Here, she answers questions to help non-scientists understand her findings about how the body supports the growth of new cells—and see how this knowledge could be applicable to developing treatments to fight the out-of-control cell growth in some forms of cancer.
Dr. Mei is also an affiliate faculty member in the Cancer Center at Illinois, an affiliate of the Personalized Nutrition Initiative, and a faculty member in the Division of Nutritional Sciences.
We hear a lot about fetal stem cells, but adult bodies also contain stem cells, which are needed to replenish the tissue in a variety of organs. How does that happen in the healthy intestine?
The cells that line the internal surface of the intestine are called intestinal epithelial cells. These cells play a critical role in the digestion of food and absorption of nutrients. The cells can be damaged by harmful components in food, such as pathogens and environmental toxicants. To make sure intestinal epithelial cells function properly, they are replaced by new intestinal epithelial cells generated by intestinal stem cells approximately every 2 to 5 days.
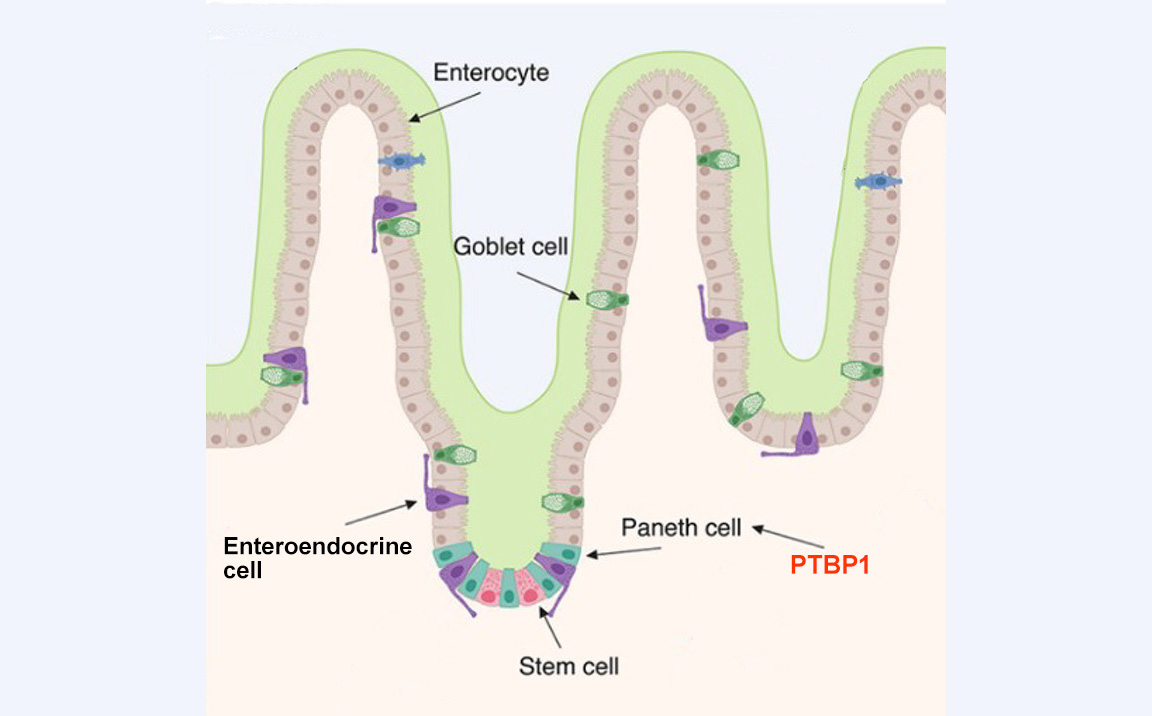
Because intestinal stem cells are critical in epithelial cell regeneration, the body develops a niche to protect them. Niche cells (called Paneth cells) not only provide growth factors to support intestinal stem cell survival and division, but they can also become stem cells if stem cells are damaged.
Our study was designed to better understand how niche cells support intestinal stem cells and compensate for their loss.
This study used a mouse model to explore the role of a particular RNA-binding protein named polypyrimidine-tract binding protein 1 (PTBP1). What did you find out about what it does? Why is that important?
We deleted PTBP1 in mouse intestinal epithelial cells to study the function of that protein. We found that PTBP1 is required for niche cells to protect intestinal stem cells.
Loss of PTBP1 in the intestinal epithelial cells results in intestinal stem cell death. Furthermore, without PTBP1, the niche cells lost the ability to become stem cells. With the intestinal epithelial cell regeneration impaired in this manner, the mice died because of a lack of nutrient and water absorption.
Our studies thus demonstrate that PTBP1 not only controls niche cells to support intestinal stem cell survival but also enables niche cells to acquire stem cell properties when intestinal stem cells are damaged.
Of particular interest, we found PTBP1 executes these roles by post-transcriptionally regulating gene function. We believe ours is the first study to demonstrate a mechanism by which niche cells are controlled by PTBP1 at the post-transcriptional level.
What is the “post-transcriptional” regulation of gene expression? Why do you study it?
DNAs in cells carry genetic information. Transcription is the process by which the information in a strand of DNA is copied into a new molecule of messenger RNA. While a lot of research focuses on the transcription stage of gene expression, the body also has ways to impact what will happen to the RNA after transcription, which is called post-transcriptional regulation.
“Post-transcriptional” regulation of gene expression is very important because it helps change gene function quickly in response to the body’s needs. For example, post-transcriptional gene regulation can generate multiple different proteins from a single gene. It can also control the timing and amount of protein production, which is critical for our body to function properly.
RNA-binding proteins are major players in executing post-transcriptional regulation. Therefore, mutations that occur in RNA-binding proteins cause many devastating human diseases, including cancers. Understanding how RNA-binding proteins control post-transcriptional regulation is crucial for understanding the causes of human diseases.
You mentioned this work was done through collaboration with other researchers on campus. How did the collaboration help to complete the study?
Good collaboration with other teams not only fills in the skill gaps within an individual lab but also provides opportunities for exchanging ideas across disciplines and learning new research skills. I believe this is crucial for success in conducting scientific research.
In this work, we studied how PTBP1 controls approximately 850 genes at different steps of post-transcriptional regulation to regulate niche cells and stem cells in the mouse intestine. Because of the large number of variables, we needed to use computational analysis to identify PTBP1 target genes and the post-transcriptional regulation provided by PTBP1 on these genes.
Dr. Auinash Kalsotra (CGD/GNDP), professor of biochemistry in the School of Molecular and Cellular Biology, has extensive experience in computational analysis of post-transcriptional regulation. By collaborating with his lab, we successfully identified the post-transcriptional regulation program controlled by PTBP1 in the intestine.
In addition, we collaborated with Dr. Jing Yang, from the Department of Comparative Biosciences, who helped us test the PTBP1 target gene using in vitro cultured cancer cells. This collaboration not only led to the new discovery of intestinal stem cell regulatory mechanisms but also provided opportunities for my lab to learn new skills that will be important for our future studies.
How might your findings lead to beneficial treatments of diseases of the intestine?
Intestinal stem cells are important for intestinal epithelial cell regeneration. Our finding has revealed novel mechanisms niche cells use to support intestinal stem cell survival and regeneration.
This finding helps to build our knowledge of stem cell biology, which is important for developing stem cell-based tissue regeneration strategies.
Furthermore, our findings may enhance our understanding of colorectal cancer stem cells, which is important for improving colorectal cancer treatment. Colorectal cancer stem cells are a subset of cancer cells in colorectal tumors that drive tumor progression and are often resistant to chemotherapy. These cells share many characteristics with intestinal stem cells, such as being capable of indefinitely dividing to generate new cells and self-renewing.
Understanding how niche cells support intestinal stem cell survival and regain stem cell properties under normal conditions may help us to develop effective therapeutic strategies that specifically kill cancer stem cells.
