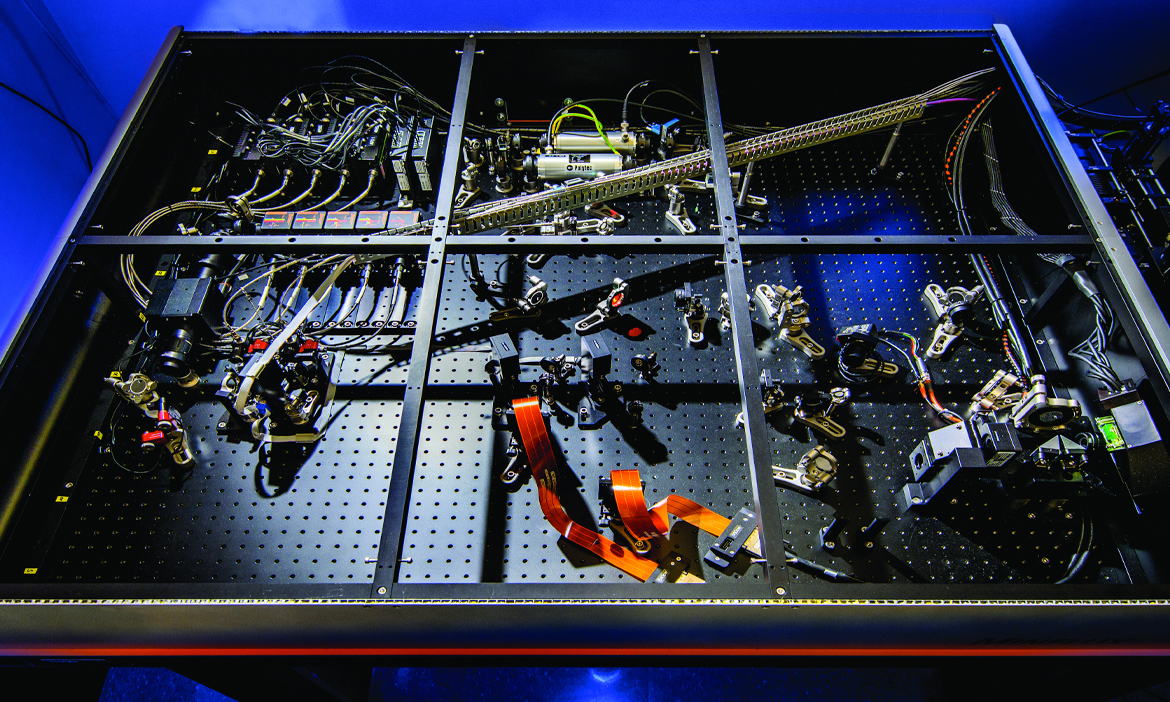
Core Facilities at the IGB is your resource for biological microscopy and image analysis. Our facilities are conveniently located, and we provide a variety of services to both IGB researchers and others in the campus community. Our facility is meant to encourage innovation – try new techniques and approaches to achieving your research goals without a significant investment in instrumentation or time.
Core Facilities at the IGB is your resource for biological microscopy and image analysis. Our facilities are conveniently located, and we provide a variety of services to both IGB researchers and others in the campus community. Our facility is meant to encourage innovation – try new techniques and approaches to achieving your research goals without a significant investment in instrumentation or time.
We offer:
- High-end equipment
- User training
- Ongoing support, including experiment design and data interpretation
- Twenty-four hour access
IGB Core Facilities is located on the concourse level of the Carl R. Woese Institute for Genomic Biology at the University of Illinois at Urbana-Champaign.
Mission
The Core Facilities at the IGB is a state-of-the-art resource for biological microscopy and image analysis. The core mission of the facility is to provide IGB faculty, as well as faculty from across campus with the tools and expertise to meet their imaging goals. In addition to providing technical assistance in acquiring and analyzing microscopy images the staff is also able to aid in designing and interpreting experiments. Check out this video to find out more about the Core Facilities.
Education and Training
The Core Facilities train over one hundred new users a year on one or more instruments and are co-hosting a bioimaging camp for middle school girls with GAMES.
Our new user procedures form guides new users through the training process. To request training (theory training is required for most microscopes including LSM 700, LSM 710, Apotome, SR-SIM, before the hands-on training), please fill out our training request form. Users have access to IGB disk space through the IGB computer and network resources group to transfer data. To request access to the IGB building, please fill out the IGB Entry Request Form.
Training pages can be found here.
Collaboration
In addition to the typical fee for training and instrument time, collaborations with the core facilities staff can be beneficial in the development of unique methods or capabilities. Publications containing work performed in the core facilities should use these guidelines for acknowledging the core facilities. Download a copy of our brochure.
The IGB Core cooperates with the following facilities on campus to provide an extensive selection of instrumentation to campus researchers:
Molecular and Cellular Biology
Roy J. Carver Biotechnology Center
A list of additional facilities can be found here.
The Illinois Roy J. Carver Biotechnology Center (CBC) and Carl R. Woese Institute for Genomic Biology (IGB) have partnered to establish an integrated suite of next generation instrumentation that is now available for users to conduct cutting-edge Omics analyses within the three-dimensional (3D) structural, compositional and formational history of any given sample. Samples can be composed of: (1) exclusively tissues and cells; (2) a combination of tissues and cells with biomineral deposits (e.g., bones, teeth, ectopic calcification); and/or (3) exclusively biomineral deposits. This new analytical pipeline begins in the IGB Core Facility, with 3D micro-CT x-ray structural scans of entire small-to-large samples at a resolution of 3-100 microns (depending on sample size, shape and composition), coupled with micron-scale Raman characterization of the mineralogical composition of embedded biomineral deposits. High-quality histology sections and petrographic thin sections can be strategically prepared and further interrogated using the suite of other cutting-edge optical and electron microscopes available within the IGB Core Facility. Analysis of these samples can then continue with the coordinated efforts of the DNA Services, Cytometry and Microscopy to Omics (CMtO), and High Performance Computing Bioinformatics (HPCBio) facilities within the CBC, which creates a unique opportunity to conduct: (1) cell cytometry analysis and sorting; (2) 10X Genomics Visium CytAssist spatial transcriptomics and proteomics from total mRNA within original 3D tissue and biomineral structure (bridging histology and genomics); and (3) 10X Genomics Chromium high-throughput or deep single cell RNA-Seq. The specific suite of applicable microscopy and omics analyses will depend on sample type, composition and preservation, as well as project goals and budgetary constraints. Users who wish to use these integrated analytical facilities can begin by arranging a consultation session with the IGB and CBC Lab Directors identified on the websites listed above.

View Gallery
1
/
10
What’s new at the Core
- The IGB Core is moving to BookitLab for instrument tracking
-
The IGB Core has purchased BookitLab for tracking instrument use, reserving instrument time and processing billing. The Core plans to setup BookitLab on the LSM 700 April 15th. The April 17th Lunch with the Core will be dedicated to introducing the BookitLab software to existing users. The plan is to transition all instrument tracking to BookitLab by early May. The IGB BookitLab portal can be found here.
Please let us know if you have any questions.
- Midwest Advanced MINFLUX and Super Resolution Workshop 2024
-
Save the Date!
The IGB Core Facilities is hosting the 2nd Annual Midwest Advanced MINFLUX and Super Resolution Workshop from May 20th to 24th. You can find updated information here.
- Lunch with the Core - Spring 2024
-
The IGB Core Facilities is hosting a Spring 2024 seminar series covering the instrumentation in the Core and some of the research taking place in the Core. The seminars are typically held from 12:00 - 1:00 pm in the Carl R. Woese Institute for Genomic Biology, Room 612 (Concourse level). Pizza will be provided to attendees. Please check the Lunch with the Core news posts and weekly emails for updates.
February 14, 2024 "Integrating Live Confocal Microscope Imagery of Plant Stomata with Gas Exchange Measurements" - Joseph Crawford, Postdoctoral Research Associate - Plant Biology
Instrument: LSM 710
Joe's talk can be found here.
February 28, 2024 "Introduction to STEDYCON: A Confocal and STED System with Super-resolution Capabilities" - Umnia Doha, Research Scientist - Core Facilities
Instrument: STEDYCON
Umnia's talk can be found here.
March 6, 2024
"X-Ray Computed Tomography in the Core Facility: Research and Manufacturing Applications of the NSI X5000" - Dr. Austin Cyphersmith, Assistant Director of Research Instrumentation - Core Facilities
Instrument: X5000
Austin's talk can be found here.
March 27, 2024 "3D Data Analysis and Segmentation Tools" - Dr. Kingsley Boateng, Assistant Director of Bioscience Research & Microscopy - Core Facilities
Instrument: Amira & Imaris Software
Kingsley's talk can be found here.
April 3, 2024 "Revolutions in Data" - Dan Davidson, Director of CNRG and Research Computing - Carl R. Woese Institute for Genomic Biology
Dan's talk can be found here.
April 10, 2024 "A solid-liquid superposition model for interfacial solvation structures" - Qian Ai, Graduate Research Assistant - Materials Science & Engineering
Zoom:
https://illinois.zoom.us/j/88982127756?pwd=ME1jZWtZd2txUDBRS3pZa2lUWmNrUT09
Meeting ID: 889 8212 7756
Password: 201556
April 17, 2024 "Introduction to BookitLab" - Dr. Glenn Fried, Director of the Core Facilities
Join Zoom Meeting
https://illinois.zoom.us/j/85934995263?pwd=eE9ZeXNuNFozNCtKdytYMTMyK3dQUT09Meeting ID: 859 3499 5263
Password: 166361April 24, 2024 "Introducing Spatial Transcriptomics to Core Facilities: A Custom Built MERFISH Microscope " - Duncan Nall, Research Scientist - Core Facilities
Instrument: MERFISH
Zoom:
https://illinois.zoom.us/j/87903107373?pwd=R1dYM2dIMmxxTVhWTGNsclNzY2ovdz09
Meeting ID: 879 0310 7373
Password: 783805
May 1, 2024
"Creating Art of Science" - Julia Pollack, Creative Program Manager - Carl R. Woese Institute for Genomic Biology
Zoom:
https://illinois.zoom.us/j/88064323223?pwd=VjJOM1drU2xVOUhHVnM0UUxIL0Q0dz09
Meeting ID: 880 6432 3223
Password: 410927
If you require special accommodations or have dietary restrictions, please email Hannah McClellan - hlm@illinois.edu.
- The STEDYCON has arrived
-
The Care Facilities has purchased a STEDYCON system from Abberior Instruments. This microscope will function as a four color confocal or a two color 2D STED microscope with 40nm resolution. For more information, please visit our STEDYCON instrument page found here.
- Dr. Reza Rajabi-Toustani has joined the IGB Core Facilities
-
Dr. Reza Rajabi-Toustani will be helping with hands-on training with the MINFLUX and other optical microscopes. Previously he was a Postdoc in Joe (Huanyu) Qiao Lab. Reza has been using the MINFLUX from the time it was installed and provided the July 2022 Image of the Month. It can be found here.
- Dr. Umnia Doha has joined the IGB Core Facilities
-
Dr. Doha will be helping with training on the optical microscopes. Umnia comes from the Taher Saif Lab in Mechanical Science and Engineering.
Location
The Core Facilities is located on the concourse level (basement) of the Carl R. Woese Institute for Genomic Biology at the University of Illinois Urbana-Champaign. The entrance is adjacent to Array Café.


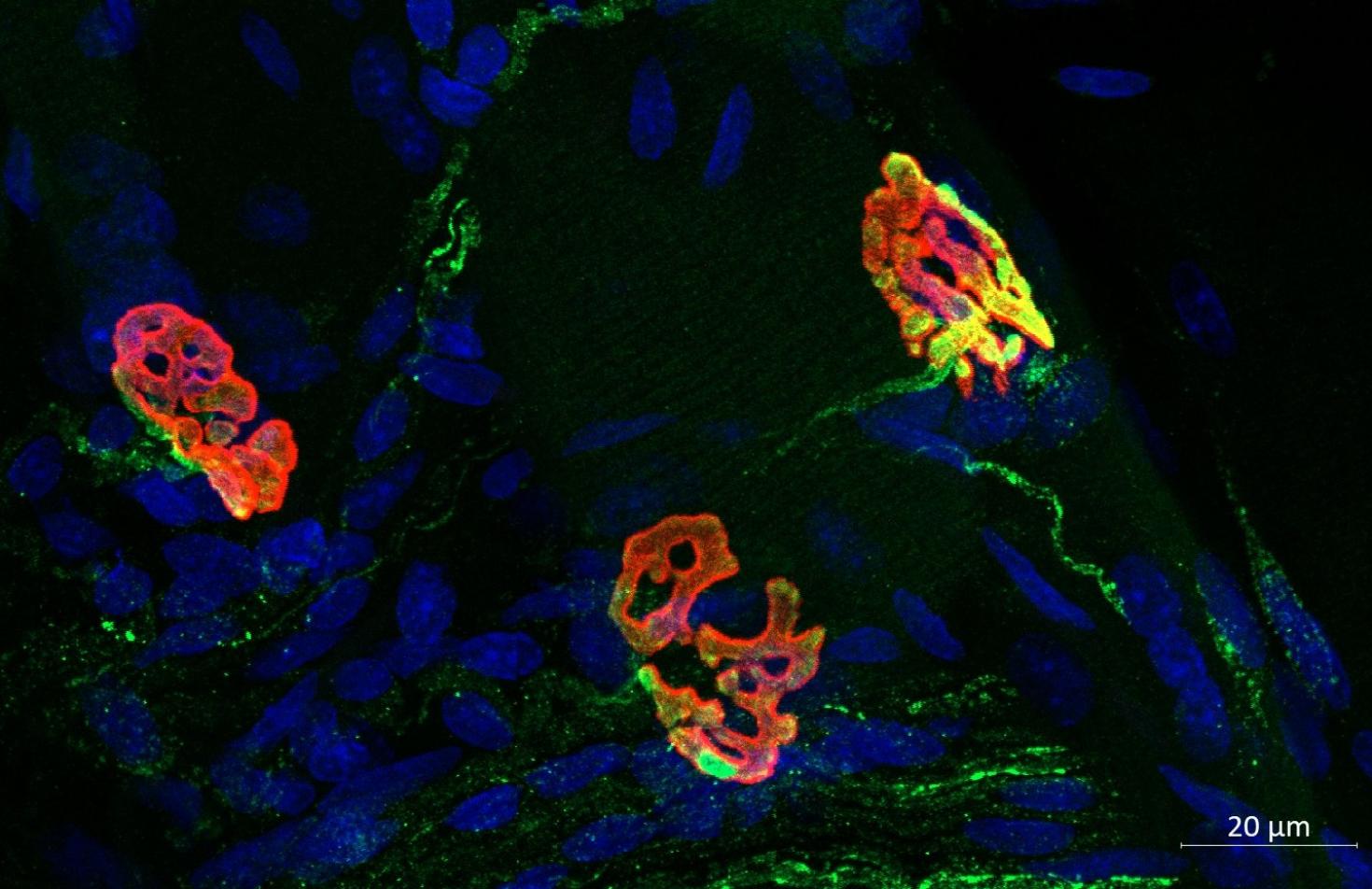
Three neuromuscular junctions (NMJs) from the diaphragm of a young male rat, illustrate the complex connections between motor neurons and muscle fibers that facilitate precise control of muscle contractions for breathing. This work was done by Sarah Asif from the Martha Gillette Lab and funded by the Chan Zuckerberg Biohub Chicago Acceleration Award. Image was taken with the LSM 900.
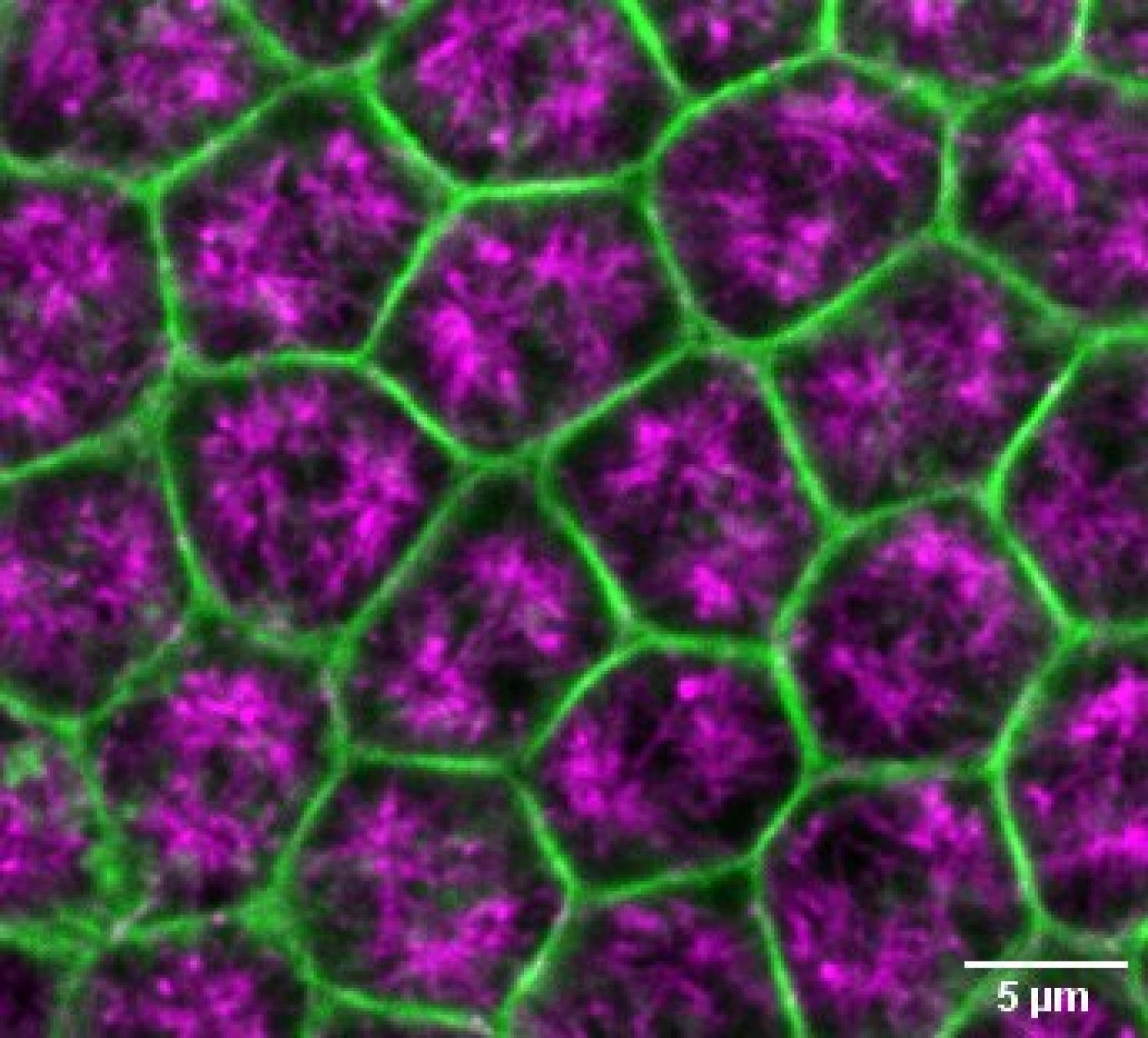

This image demonstrates a surface view of Drosophila embryo undergoing cellularization, depicting microtubule in magenta and Actin in green. The objective of this study is to investigate the changes in microtubule-actin interactions in the microvillar membrane reservoir during early embryonic morphogenesis in an intact tissue. The image was captured using STEDYCON microscope at the Core Facilities of the Carle R. Woese Institute for Genomic Biology.
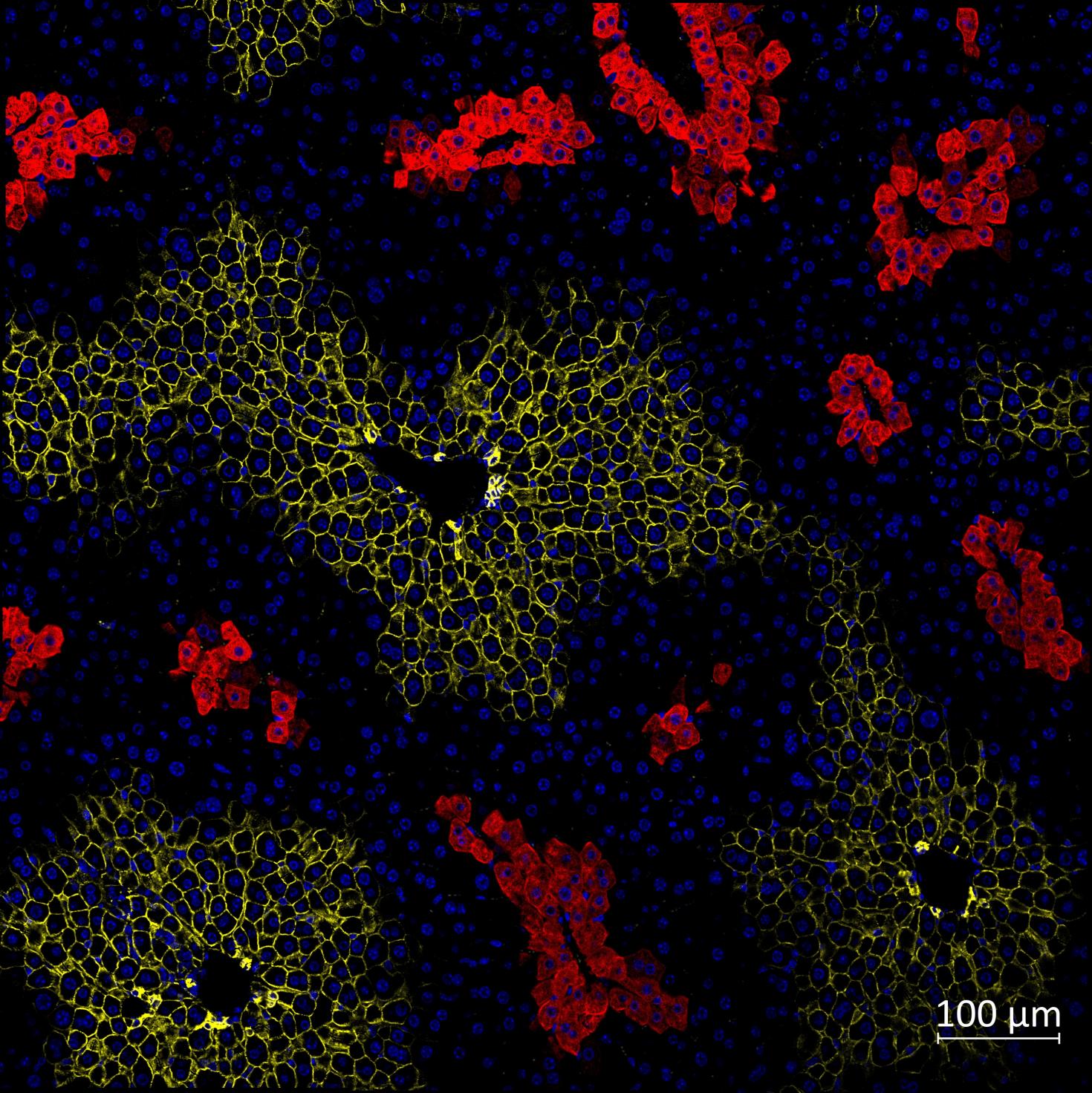
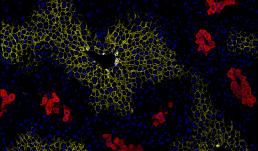
Immunofluorescence of mice liver sections with nuclei (blue) and zonal markers E-cadherin (periportal, yellow) and glutamine synthetase (pericentral, red). The liver helps maintain metabolic processes, hormone production, nutrient storage, and detoxification. This happens when the oxygen and nutrient-rich blood flow from the periportal vein to the pericentral vein in the liver lobule, the functional unit of liver. As a result, the blood flow creates a gradient of oxygen, nutrients and signaling molecules, creating three different zones in the liver lobule. These zones have different functions, and they express different proteins depending on their functions. Here, we have used Glutamine synthetase (red) to view the pericentral zone and E-cadherin (yellow) for the periportal zone to study the zonation.
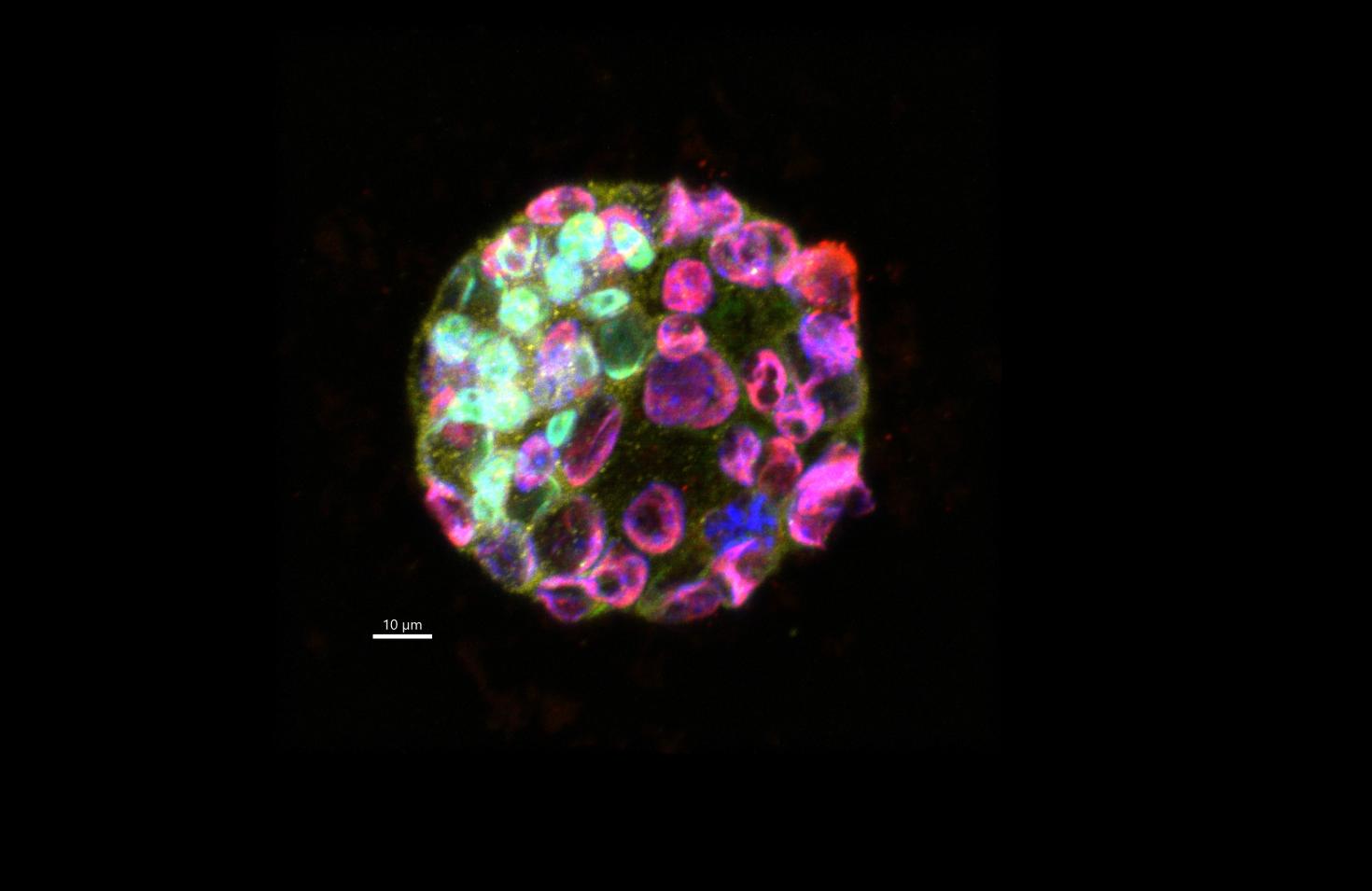
The proteins we evaluated in this embryo are GATA6 (green), CDX2 (red) and OCT4 (yellow). DAPI is shown in blue.
This embryo is part of a study to identify the effects of DEHP, which is a plasticizer whose chemical structure closely resembles hormones and is believed to be an endocrine disruptor. Previous studies have shown that it affects embryo development and implantation in the uterus. These proteins are important in cell differentiation to give rise to different organs in future stages. For this reason we want to evaluate its expression in the presence of DEHP and to understand the mechanism of its effect on embryo development and embryo implantation.
Image taken on LSM880.
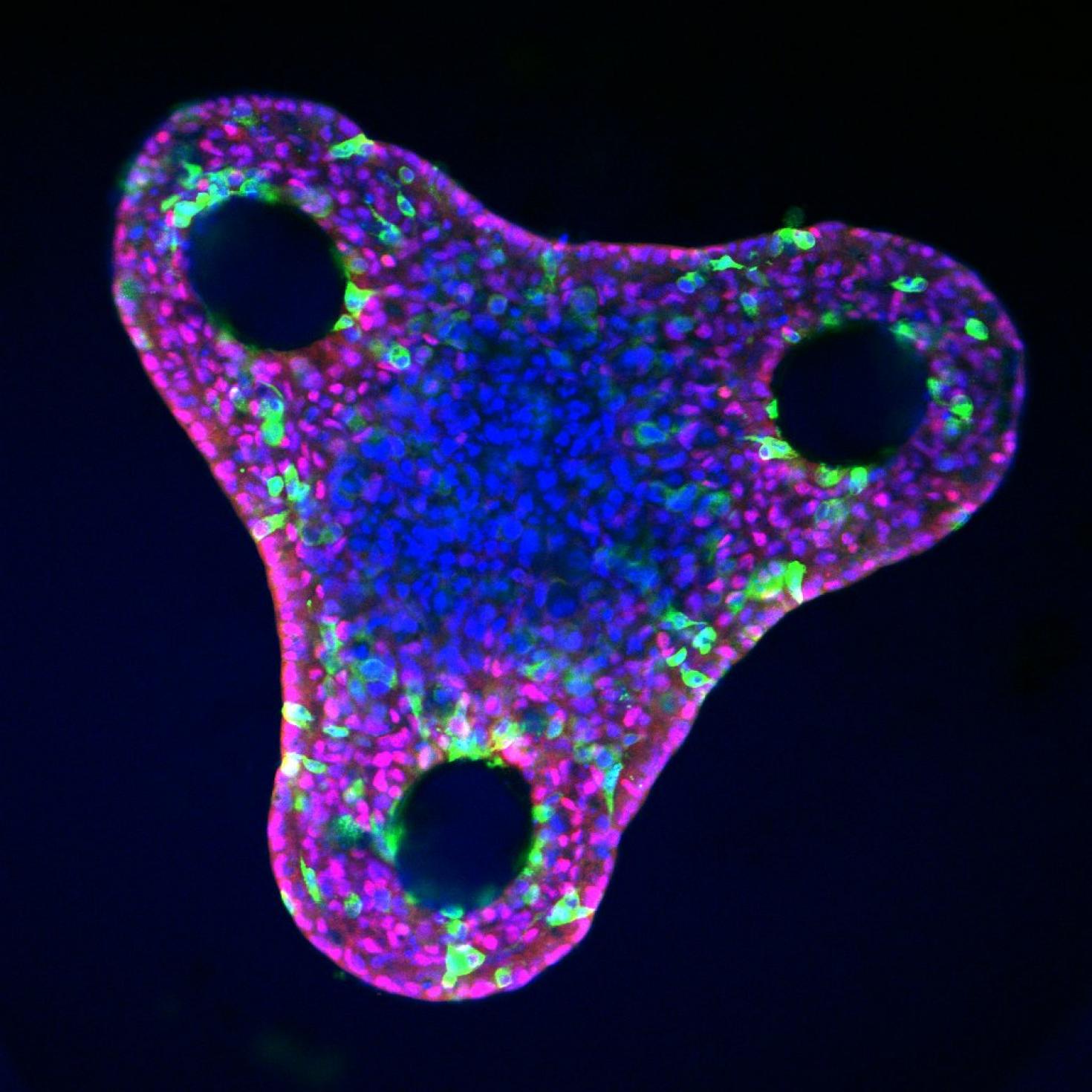
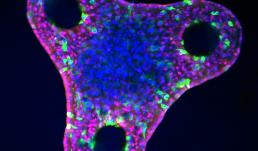
Liver organoid after differentiation in a triangular microwell. Varying organoid shape changes mechanical cues and can influence liver cell fate and patterning. The red stain is HNF4a, a hepatocyte marker, green is OPN, a biliary cell marker, and blue is DAPI, a nuclear DNA stain. Images were taken with the LSM 700.
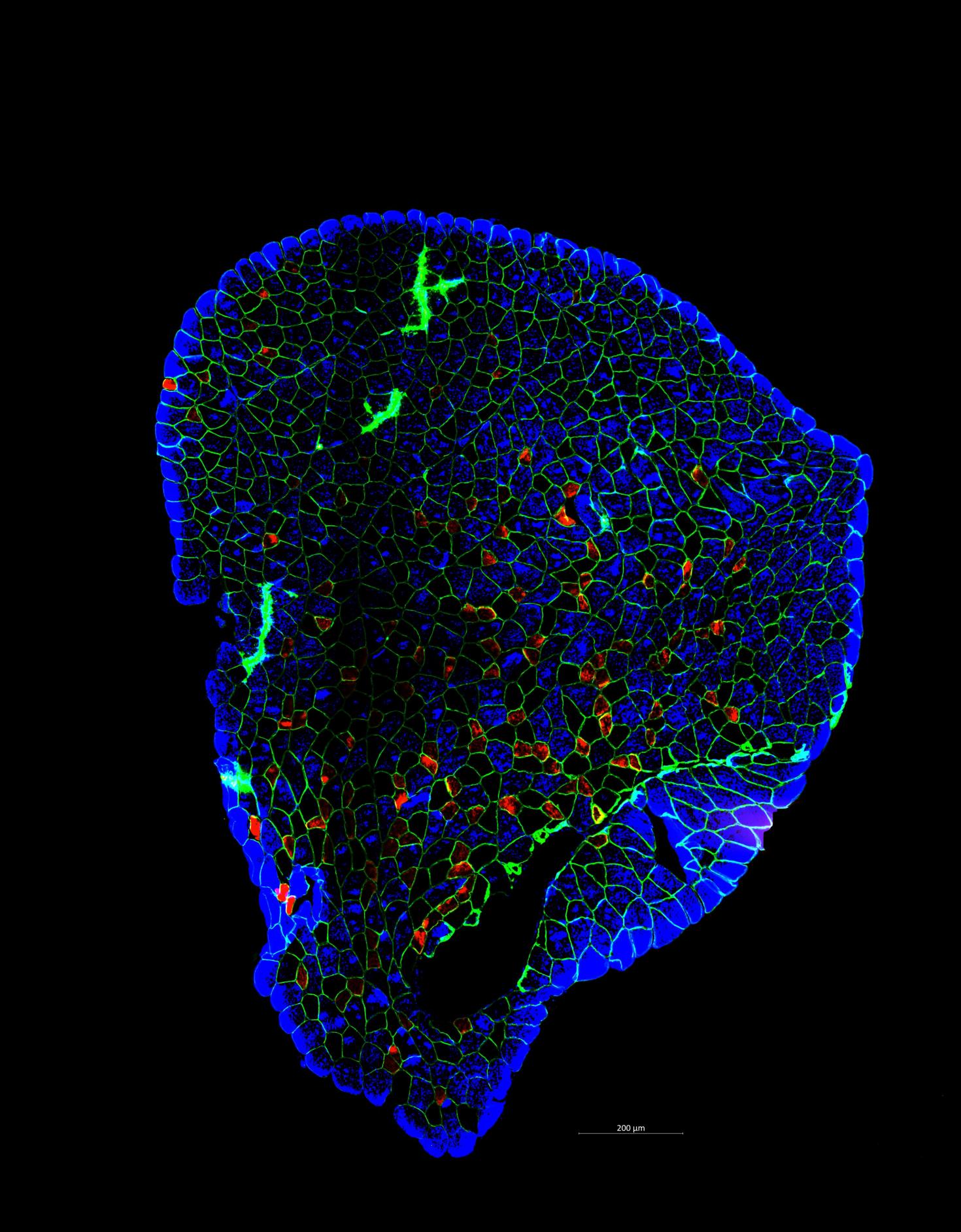
A plantaris muscle stained for individual fiber types. Fibers are "outlined" in green (stained for dystrophin). While the individual fibers are stained based on myosin heavy chain. Type IIa is red, IIx is black, and IIb is blue.
Instrument used: Axioscan
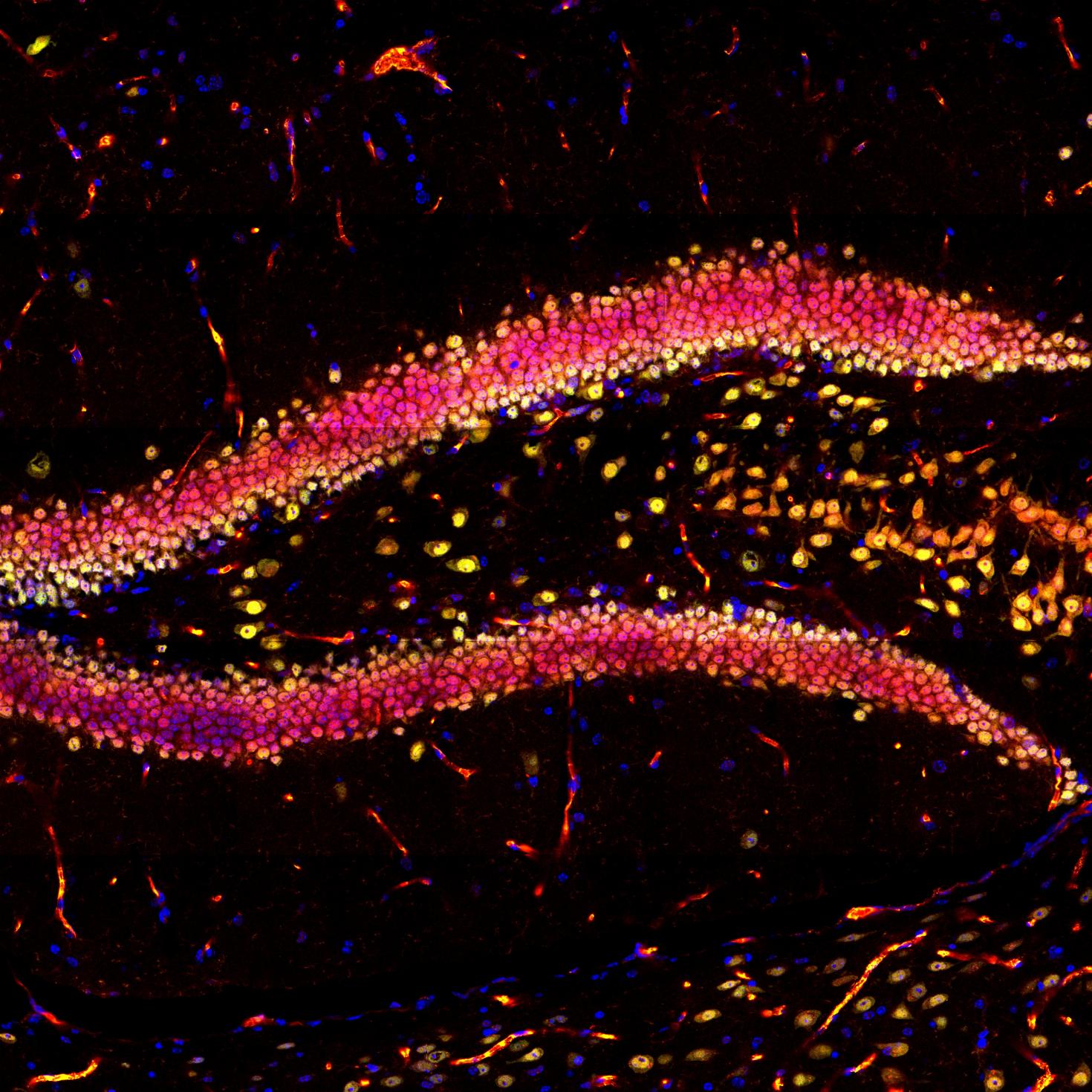
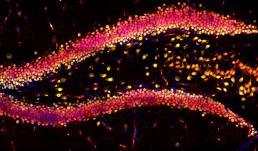
This image showcases the hippocampal region in a mouse brain slice, highlighting DAPI in blue, NeuN in green, and YAP in red using immunofluorescence. The objective of this research project is to investigate the impact of physical exercise on neurogenesis in the hippocampus. The images were captured using the Zeiss LSM 700 confocal microscope at the Core Facilities of the Carle R. Woese Institute for Genomic Biology.